Adverse Events Following Immunization (AEFI) Secretariat
While vaccines are generally safe, as with other medicines, adverse events may rarely occur, following use of vaccines. Apprehensions around risks associated with vaccines are a barrier to acceptance of vaccines. A functional AEFI surveillance system ensures vaccines are safe by monitoring AEFIs reported and investigated in districts.
The AEFI Secretariat was set up in ITSU in 2012 with the objective of having a group of dedicated personnel at national level, focussing only on vaccine safety surveillance. Adverse events following use of any vaccine, whether in the Universal Immunization Programme (UIP) or private sector, paediatric vaccines or vaccines used in adults or for international travel, etc. should be reported to the AEFI surveillance system. There is no time limit after vaccination for reporting adverse events.

AEFI is any untoward medical occurrence that follows immunization and which does not necessarily have a causal relationship with usage of vaccine. All AEFIs reported are suspected adverse events which may/may not be caused due to vaccine. Any death/hospitalization/disability/birth defect/events occurring in clusters or suspected by a vaccine recipient/relative/community member/media person/health worker due to vaccination should be reported as an adverse event and investigated.
Investigations of serious and severe AEFI cases will help in conducting assessments to understand the cause of AEFI. AEFIs are classified as causally related to the vaccine or vaccination process (vaccine product related, vaccine quality defect related, anxiety reactions, immunization error related) or coincidental (not related to vaccine or vaccination process, due to something other than the vaccine), indeterminate or unclassifiable.
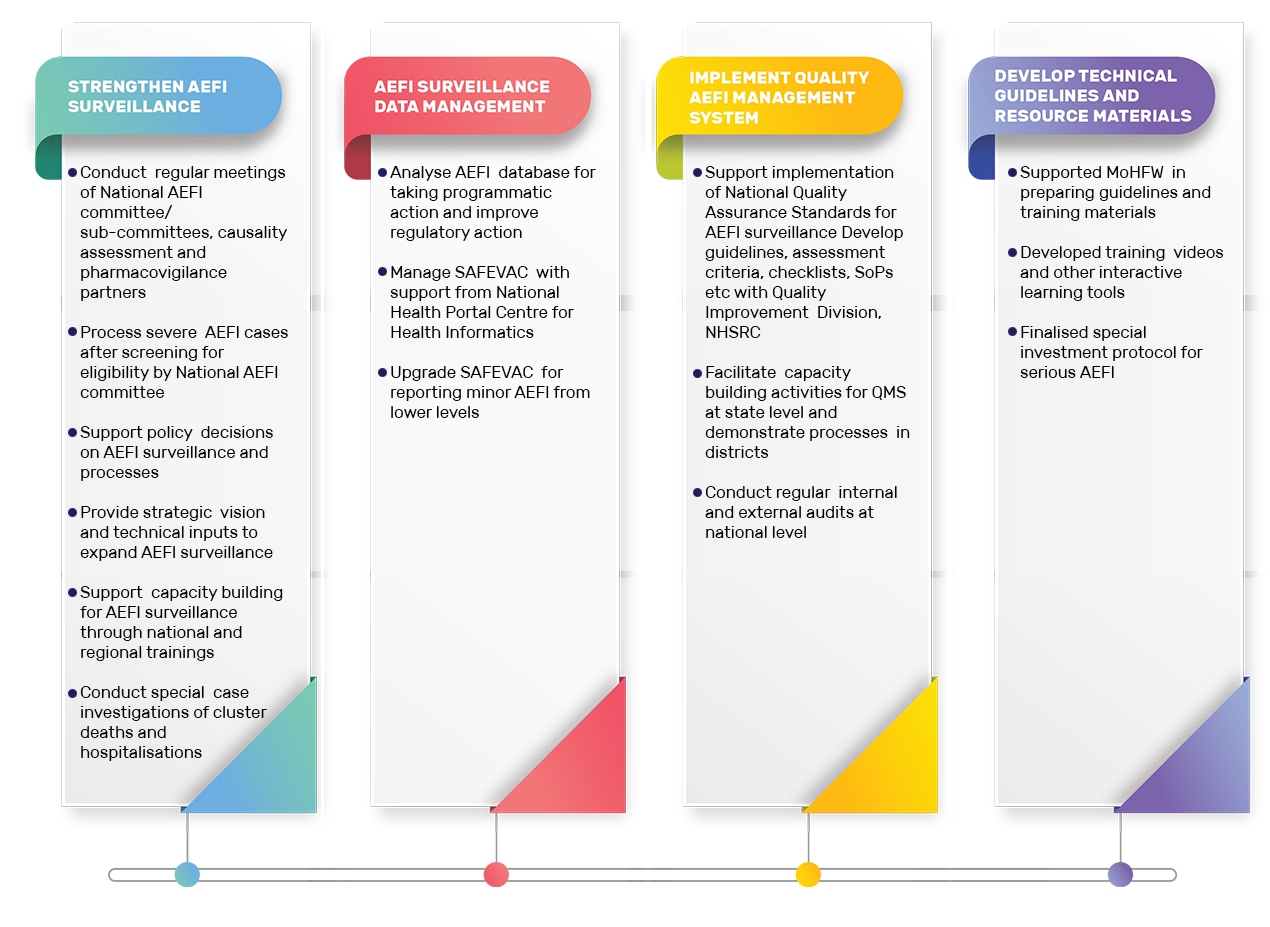
Core functions of AEFI Secretariat
AEFI Secretariat at ITSU manages AEFI data (adverse events reported as hospitalizations, deaths, etc. following vaccination), follows up with states for investigations, and facilitates causality assessments of cases at national level. The Secretariat provides strategic vision to improve AEFI surveillance and vaccine safety under overall guidance of the National AEFI Committee and National AEFI Technical Collaborating Centre at Lady Hardinge Medical College (LHMC), New Delhi. It supports MoHFW in taking policy decisions related to AEFI surveillance and vaccine safety. The national AEFI surveillance guidelines are developed and updated by the AEFI Secretariat with support of WHO-India Country Office. The last national AEFI surveillance operational guidelines were updated in 2015.
Four national sub-committees (causality assessment, investigation, media and communication and laboratory subcommittee) monitor and support activities related specifically to domains allotted to them. The causality assessment sub-committee meets once a month and assesses serious and severe AEFI cases. Causality assessments are conducted at national level as per globally accepted guidelines developed by WHO (Causality Assessment of an Adverse Event Following Immunization (AEFI), WHO).
Results of these cases are approved by National AEFI committee which meets once a quarter. Results of causality assessment of 5140 serious and severe AEFI cases following routine vaccinations are available here.
The investigation subcommittee guides and assists AEFI Secretariat and national AEFI committee to conduct quality investigations and is involved in conducting special investigations.


SoPs for special investigation of AEFIs, especially those occurring in clusters have been developed by use by investigating teams at national, state and district levels. Reports of special investigations conducted by central teams are available here.
A team of data managers downloads AEFI data from AEFI reporting and management software (SAFEVAC – Surveillance and Action For Events following Vaccination) every day and analyzes trends on weekly, monthly and annual basis. The AEFI Secretariat has a repository of approximately 13,000 serious and severe AEFI cases reported since 2012.
Support is provided to states and districts to improve AEFI reporting, investigations and causality assessments through four Senior AEFI Zonal Consultants, who visit the states and ensure state AEFI committee meetings are held regularly, provide feedback and facilitate causality assessment of AEFI cases.
State and district AEFI committees are functional in all states and UTs. They ensure AEFI surveillance activities are conducted as per national AEFI surveillance guidelines in states and districts.
A ranking system for states and UTs is in place, using indicators related to reporting, investigation, causality assessments and conduct of state AEFI committee meetings. These are shared with states on quarterly basis to encourage healthy competition. A State Guidance Document for AEFI Surveillance Processes has activities described as SoPs in a sequential manner to undertake activities to improve AEFI surveillance activities. A similar document has been developed for activities at district level (Demonstration of Model AEFI Surveillance Processes).
AEFI Secretariat also works with experts to bring out guidelines to prevent and manage expected AEFIs. Anaphylaxis reactions are expected following any vaccination. It is difficult to predict who will have an anaphylaxis reaction. In order to manage anaphylaxis reactions at the session site, the AEFI Secretariat facilitated development of anaphylaxis kits for use at session site by vaccinators in case of suspected anaphylaxis reactions. Anaphylaxis guidelines and film for training purposes were developed and disseminated.
Similarly, managing vaccination session sites, preventing over-crowding and proper communication of processes before vaccination will prevent occurrence of anxiety reactions which may occur in clusters among adolescents. To reduce chances of overdosage and prevention of inadvertent administration of tablets other than paracetamol to infants and children post vaccination, guidelines were developed and implemented for dispensing syrup paracetamol in vaccination sessions in place of tablets.
A quality management system has been initiated in the country for AEFI surveillance in collaboration with the Quality Improvement Division of the National Health Systems Resource Centre, New Delhi. This system is expected to help improve processes of AEFI surveillance in districts and states. Processes are recorded and indicators benchmarked at session sites/primary health centers (PHC), district, state and national levels with regular internal audits and external audits leading to certification. The National Quality Assurance Standards for AEFI surveillance was developed in 2016. Currently efforts are on to implement the Quality Management System in 26 states/UTs.
Specific resource materials, such as AEFI media communication protocol have been developed to help communicate to media and community in case of crisis situations related to vaccine safety such as death or cluster of cases reported as adverse events. The team works closely with communication team in ITSU, UNICEF and MoHFW to develop key messages related to advocacy and behavior change.
Regular trainings on AEFI surveillance are conducted in collaboration with WHO-NPSP for health care staff such as District Immunization Officers (DIO), Medical Officers and vaccinators are part of immunization trainings for these cadre. In addition, regional and state workshops are held for conducting causality assessments (for state AEFI committee members) and for improving reporting and investigations (for state and district immunization officers). Trainings on how to report cases on SAFEVAC are conducted for state and district immunization officers and data entry operators.
A training film (Renuka: the health worker) depicting entire process of reporting, investigations and roles of health workers, PHC doctors, DIO and the district AEFI committee is available in English, Hindi and four major languages of south India. Presentations on AEFI surveillance for use in training medical officers are also available along with Case Reporting Forms, Case Investigation Forms, verbal autopsy forms for adults and Lab Request Forms.
AEFI Secretariat monitors adverse events reported following any new vaccines (rotavirus vaccine, MR and PCV) introduced in UIP for at least a year following introduction. This is important because some vaccines have been used first time globally at such large scale. AEFI Secretariat has contributed to a White Paper on Safety of Rotavirus Vaccine in India.
Sharing of data and information related to vaccine safety is done as per protocol nationally (to Drug Controller General of India, Central Drugs Standard Control Organization, Indian Pharmacopeia Commission, National Technical Advisory Group on Immunization, etc.).
COVID 19 activities related to vaccine safety
In pre COVID-19 phase, the focus has been in strengthening AEFI surveillance for routine immunization. Since October 2020, the AEFI Secretariat worked on ensuring that AEFI surveillance was modified to capture AEFIs reported following use of novel vaccines in adults. Steps taken to strengthen AEFI reporting mechanism in the country are:
- Enabling Integration of CoWIN and SAFEVAC software to provide online platform for vaccinators/health worker and DIO to report AEFIs (including minor AEFI cases) occurring following COVID-19 vaccinations.
- Necessary guidance issued to states/UTs to strengthen AEFI surveillance for COVID-19 vaccinations such as inclusion of medical specialists, neurologists, cardiologists, obstetrician-gynaecologists, etc. in state and district AEFI committees, collaborating with state medical colleges to function as state AEFI technical collaborating centre, cascade trainings of health work force (including state and DIOs and medical officers) on AEFI surveillance, hiring of state AEFI consultants, expansion of reporting network to include private health care facilities and large hospitals for sensitizing and reporting AEFIs, etc. Guidance issued to states/UT for death investigations – conduct of post mortems by multidisciplinary panel in medical colleges/district hospitals, videography of post mortem, RTPCR test etc.
- Rapid review of reported serious/severe AEFI cases was done to understand trends/possible safety issues following use of COVID-19 vaccines. Advisory issued for Thrombosis with Thrombocytopenia Syndrome (TTS) was shared with states. Read more
- Timelines for submission of reporting, investigation, causality assessment forms were reduced to enable quick assessment of causality by trained medical experts. Causality assessments of serious/severe AEFI cases are expedited through frequent meetings of national AEFI committee and causality assessment sub-committees. Approved causality assessment results of 1099 cases are uploaded on MoHFW website for general public. Read more
- Signals are previously unknown reported adverse events suspected to be caused due to a particular vaccine requiring in-depth assessment to see if these could actually be causally due to the vaccine. Framework for systematic processes to identify, assess and manage such signals is being developed and will be implemented soon. This will be a collaborative effort with support of Central Drugs Standards Control Organization (CDSCO) and Pharmacovigilance Programme of India (PvPI) which has the expertise of implementing the same for drugs.
- Active vaccine safety surveillance system in the form of sentinel surveillance for Adverse Events of Special Interest following COVID-19 vaccination is being implemented to complement the passive AEFI surveillance system. A network of 18 sentinel sites in medical colleges across India is being used to collect information on select events. ITSU is supporting other partners and research organizations to conduct active AEFI surveillance studies related to COVID-19 vaccinations such as Cohort Event Monitoring/sentinel surveillance studies.
- Sharing of information with national drug regulators and international organizations such as WHO and Global Advisory Committee on Vaccine Safety (GACVS), etc.
- Supporting MoHFW to respond to media queries, parliamentary questions, legal notices, etc.